Electrolysis could be a promising possibility for carbon-free chemical element production from renewable and nuclear resources. Electrolysis is that the method of victimization of electricity to separate water into chemical elements and gas. This reaction takes place associate exceedingly|in a very} unit known as an electrolyzer. Electrolyzers will target size from little, appliance-size instrumentality that’s well-suited for small-scale distributed chemical element production to large-scale, central production facilities that would be tied on to renewable or alternative non-greenhouse-gas-emitting sorts of electricity production.
Schematic of a chemical compound solution membrane electrolyzer
How will it Work?
Like fuel cells, electrolyzers incorporate an Associate in Nursing Associate in Nursingode and a cathode separated by a solution. totally different electrolyzers perform in several ways that, chiefly thanks to the various form of solution material concerned and therefore the ionic species it conducts.
Polymer Electrolyte Membrane Electrolyzers
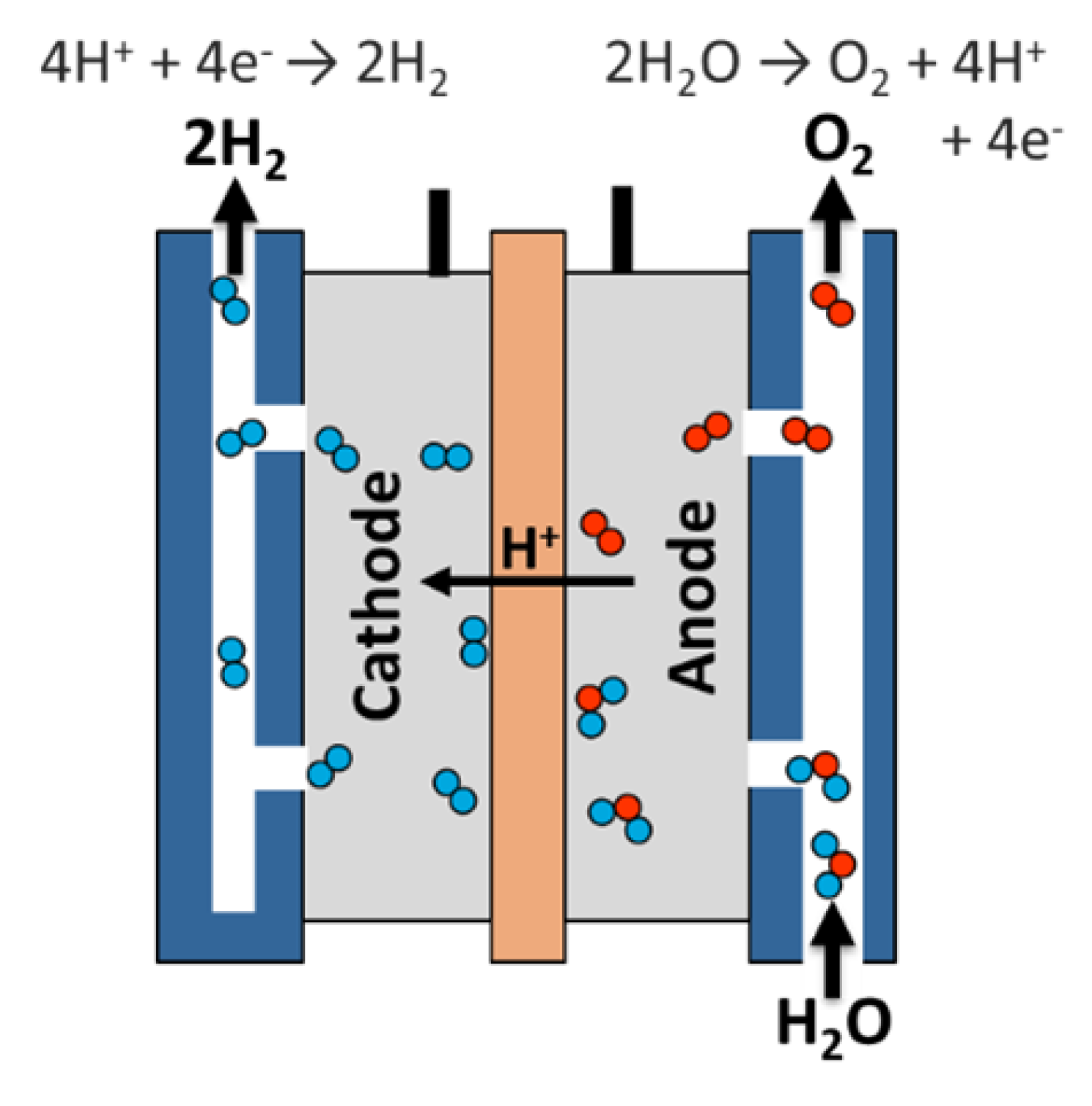
In a chemical compound solution membrane (PEM) electrolyzer, the solution could be a solid specialty plastic material.
- Water reacts at the anode to create gas and charged chemical element ions (protons).
- The electrons flow through Associate in Nursing external circuit and therefore the chemical element ions by selection move across the PEM to the cathode.
- At the cathode, chemical element ions mix with electrons from the external circuit to create chemical element gas. Anode Reaction: 2H2O → O2 + 4H+ + 4e- Cathode Reaction: 4H+ + 4e- → 2H2
Alkaline Electrolyzers
Alkaline electrolyzers operate via the transport of hydroxide ions (OH-) through the solution from the cathode to the anode with the chemical elements being generated on the cathode facet. Electrolyzers employing a liquid basic resolution of Na or hydrated oxide because the solution is commercially accessible for several years. Newer approaches victimization solid basic exchange membranes (AEM) because the solution ar showing promise on the science laboratory scale.
Solid Oxide Electrolyzers
Solid chemical compound electrolyzers, use a solid ceramic material because the solution that by selection conducts charged gas ions (O2-) at elevated temperatures generates chemical elements in a very slightly totally different approach.
- Steam at the cathode combines with electrons from the external circuit to create chemical element gas and charged gas ions.
- The gas ions undergo the solid ceramic membrane and react at the anode to create gas gas and generate electrons for the external circuit.
Solid chemical compound electrolyzers should operate at temperatures high enough for the solid chemical compound membranes to perform properly (about 700°–800°C, compared to PEM electrolyzers, that operate at 70°–90°C, and industrial basic electrolyzers, which usually operate at but 100°C). Advanced lab-scale solid chemical compound electrolyzers supported proton-conducting ceramic electrolytes are showing promise for lowering the operating temperature to 500°–600°C. The solid chemical compound electrolyzers will effectively use heat accessible at these elevated temperatures (from varied sources, as well as nuclear energy) to decrease the quantity of voltage required to provide chemical elements from water.
Why is this Pathway Being Considered?
Electrolysis could be a leading one|chemical element|element|gas} production pathway to realize the one|chemical element|element|gas} Energy Earth shot goal of reducing the price of fresh one|chemical element|element|gas} by eightieth to $1 per 1 metric weight unit in 1 decade (“1 1 1”). chemical element created via electrolysis may end up in zero gas emissions, reckoning on the supply of the electricity used. The supply of the desired electricity—including its price and potency, still as emissions ensuing from electricity generation—must be thought about once evaluating the advantages and economic viability of chemical element production via electrolysis. In several regions of the country, today’s facility isn’t ideal for providing the electricity needed for electrolysis due to the greenhouse gases free and therefore the quantity of fuel needed thanks to the low potency of the electricity generation method. chemical element production via electrolysis is being pursued renewable (wind, solar, hydro, geothermal) and energy choices. These chemical element production pathways end in nearly zero gas and criteria waste product emissions; but, the assembly price has to be cut considerably to be competitive with additional mature carbon-based pathways like fossil fuel reforming.
Potential for synergism with renewable energy power generation
Hydrogen production via electrolysis might provide opportunities for synergism with dynamic and intermittent power generation, which is characteristic of some renewable energy technologies. for instance, although the price of wind generation has continued to drop, the inherent variability of wind is an Associate in Nursing impediment to the effective use of wind generation. chemical element fuel and wattage generation may well be integrated at a power plant, permitting flexibility to shift production to best match resource handiness with system operational wants and market factors. Also, in times of excess electricity production from wind farms, rather than curtailing the electricity as is often done, it’s potential to use this excess electricity to provide chemical elements through electrolysis.
It is necessary to notice…
Today’s grid electricity isn’t the perfect supply of electricity for electrolysis as a result of most of the electricity is generated by victimization technologies that end in gas emissions and are energy-intensive. Electricity generation victimization renewable or energy technologies, either break free the grid or as a growing portion of the grid combine, could be a potential choice to overcome these limitations for chemical element production via electrolysis.
The U.S. Department of Energy et al continue efforts to bring down the price of renewable-based electricity production and develop additional economical fossil-fuel-based electricity production with carbon capture, utilization, and storage. Wind-based electricity production, for instance, is growing quickly within us and globally.
Research Focuses On Overcoming Challenges
Meeting the chemical element Shot clean chemical element price target of $1/kg H2 by 2030 (and an interim target of $2/kg H2 by 2025) through improved understanding of performance, cost, and sturdiness trade-offs of electrolyzer systems beneath foretold future dynamic operational modes victimization CO2-free electricity.
Reducing the cost of capital of the electrolyzer unit and therefore the balance of the system.
Improving energy potency for changing electricity to chemical elements over a large variety of operational conditions.
Increasing understanding of electrolyzer cell and stack degradation processes and developing mitigation ways to extend operational life.

